Given:
The mass of the substance is m = 100 g
The temperature change is
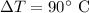
The heat provided to the substance is Q = 963 cal
To find the heat required by the same substance to raise the temperature of the substance by 90 degrees Celsius of mass m' = 300 g
Step-by-step explanation:
First we need to find the specific heat of the substance by the formula
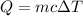
Substituting the values, the specific heat of the substance will be
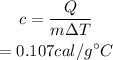
The heat required by the same substance of mass m' to raise the temperature by 90 degrees Celsius will be
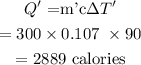
Final Answer: The heat required by the substance of mass 300 g to raise the temperature by 90 degrees Celsius is 2889 calories.