Answer: Option (C) is the correct answer.
Step-by-step explanation:
Ions are the species that form when a neutral atom either gains or loses an electron.
For example, atomic number of beryllium is 4 and its electronic distribution is 2, 2. So, in order to attain stability by losing its valence electrons Be will form a
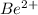 ion.
ion.
Whereas atomic number of helium is 2 and its electronic configuration is
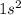 .
.
Since, it has completely filled octet so, it will neither gain or lose an electron as it is stable in nature. Hence, it will not form ions.
Thus, we can conclude that the atoms He element will NOT typically form ions.