Answer: Option (B) is the correct answer.
Step-by-step explanation:
Strong acids are the acids which completely dissociate in a solution of water. Similarly like strong acids, strong bases also dissociate completely into a solution of water.
For example,
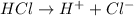
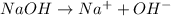
Also, there is little or no reverse reactions when strong acids and bases completely dissociate into ions.
Thus, we can conclude that strong acids and strong bases have in common that they both dissociate completely, with little or no reverse reactions.