Answer: The number of oxygen molecules produced are 1.
Step-by-step explanation:
Water is formed by the combination of hydrogen and oxygen atoms.
Water splits into 2 types of molecules when electricity is applied.
The balanced chemical equation for the splitting of water into its molecules follows:
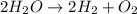
By Stoichiometry of the reaction:
2 moles of water splits to form 2 moles of hydrogen molecule and 1 mole of oxygen molecule.
Or,
2 molecules of water splits to form 2 molecules of hydrogen and 1 molecule of oxygen.
Hence, the number of oxygen molecules produced are 1.