a) Balance the equation
To balance the equation we must count the atoms of each element on both sides of the reaction. In the following figure, I will write the number of atoms of each element and I will update it as I explain it to you.
We see that we have three carbon atoms in the reactants, we must put the coefficient 3 in front of the CO2 molecule to have the same amount. I will update the figure.
Now we will balance the hydrogen, we have 6 hydrogens in the reactants. To have 6 in the products we place the coefficient 3 in front of H2O.
Finally, we balance the oxygen atoms. We have 9 oxygens in the reactants, we put the coefficient 4 in the molecule O2 to have 8 oxygen atoms, plus the oxygen of the C3H6O molecule we will have 9 in total.
Now we have the same number of atoms of each element on both sides of the reaction, the equation is balanced and will be equal to:
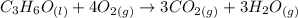
b) Heat of the reaction
They give us the heat of reaction per mole of acetone that reacts. We must calculate the moles of acetone that are contained in 155g and multiply this value by the given heat. The molar mass of acetone is 58.08g/mol.
Moles of acetone:
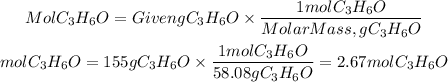
Heat of the reaction:
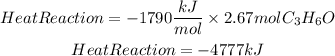
If react 155g of acetone the heat released will be -4777kJ