Given:
The initial temperature of the silver is
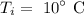
The final temperature of the silver is
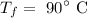
The heat added is Q = 1 kcal = 1000 cal
The specific heat of silver is
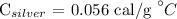
Required: The mass of the silver.
Step-by-step explanation:
The mass of the silver can be calculated by the formula
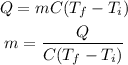
On substituting the values, the mass will be
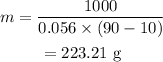
Final Answer: The mass of the silver is 223.31 g