Answer: By measuring its density which should come out to be
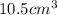 at
at
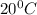
Explanation:
Density is defined as the mass contained per unit volume.
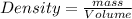
Density of a substance is an intensive property and it does not depend on the amount of matter contained in the substance. It is specific of a compound and thus can be used to know the purity of substances.
Density of silver is
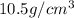 at
at
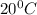 .
.
Thus if density comes out to be different from the standard value , it means the silver is impure.