Answer: The mass of silver atoms is 99.78 grams.
Step-by-step explanation:
To calculate the number of moles, we use the equation:
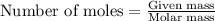 .....(1)
.....(1)
For boron:
Given mass of boron = 10.0 g
Molar mass of boron = 10.81 g/mol
Putting values in equation 1, we get:
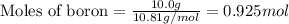
As, the number of atoms of silver are same as the number of atoms of boron. This means that number of moles of boron will be same as number of moles of silver.
Now, calculating the mass of silver atoms by using equation 1, we get:
Molar mass of silver = 107.87 g/mol
Moles of silver = 0.925 moles
Putting values in equation 1, we get:
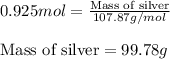
Hence, the mass of silver atoms is 99.78 grams.