Answer:
225.70 grams is the mass of a block of osmium.
Step-by-step explanation:
Density of the substance is defined as mass of the substance present in a unit volume of the substance.
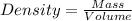
Density of the metal =
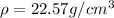
Mass of the block of an osmium metal = M
Volume of the block of an osmium metal = V
Block is in the shape of cuboid with dimension given as:
Length of the block = l = 1.0-0 cm
Breadth of the block = b = 4.00 cm
Height of the block = h = 2.50 cm
Volume of the block =

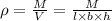
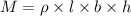
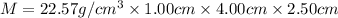
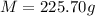
225.70 grams is the mass of a block of osmium.