Step-by-step explanation:
As molarity is the number of moles present in liter of solution. So, convert given volume into liter as follows.
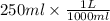 (as 1 L = 1000 ml)
(as 1 L = 1000 ml)
= 0.25 L
Therefore, number of moles of solute present will be calculated as follows.
Molarity =
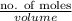
6.00 M =
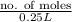
no. of moles = 1.5 mol
Hence, number of moles of solute in 250.0 ml of 6.00 M NaOH is 1.5 mol.
Now, calculate the mass of solute as follows.
No. of moles =
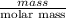
1.5 mol =
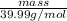
mass = 59.98 g
Thus, we can conclude that mass of solute in 250.0 ml of 6.00 M NaOH is 59.98 g.