1) Compound: Copper (I) nitrate.
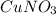
2) Convert molecules of CuNO3 to moles of CuNO3.
Avogadro's number is 6.022*10^23.
1 mol is equal to 6.022*10^23 molecules.
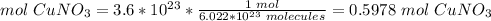
3) Convert moles of CuNO3 to grams of CuNO3.
The molar mass of CuNO3 is 125.5509 g/mol.
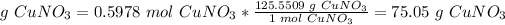
3.6*10^23 molecules of copper (I) nitrate are equal to 75.05 g CuNO3.
.