Step-by-step explanation:
A water molecule contains two hydrogen atoms bonded with an oxygen atom. That is, formula of water is
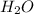 .
.
Since there is high difference in electronegativity of both hydrogen and oxygen. Thus, there will be uneven distribution of charge on a water molecule as hydrogen will have a positive charge whereas oxygen will have a negative charge.
This will also make the water molecule polar in nature.