Answer:
Step-by-step explanation:
First, let's write the chemical equation:
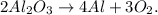
We want to know how many moles of Al2O3 we require to produce 95.4 moles of O2. You can note that in the chemical equation we have 2 moles of Al2O3 reacted that produces 3 moles of O2, so we can state a rule of three, like this:
