Given:
Specific volume = 1.188 m³/kg
Pressure, p = 150 kPa
Temperature, T = 120 C
The mass density is
ρ = 1/(1.188 m³/kg) = 0.8418 kg/m³
The ideal gas law is
pV = nRT
where
V = volume,
p = pressure,
n = numbe pf moles,
T = temperature
R = 8.314 J/(mol-K), the gas constant
The molar specific volume is
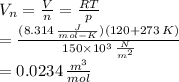
Answer:
The molar specific volume is 0.0234 m³/mol.
The density is 0.842 kg/m³