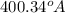Answer: The boiling point of water is
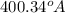
Step-by-step explanation:
To convert the units of temperature, we use the equation:
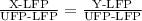
where,
X = temperature in
Y = temperature in
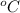
LFP = lower fixed point
UFP = upper fixed point
LFP of
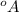 is 0°A
is 0°A
UFP of
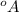 is 100°A
is 100°A
LFP of
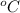 is -77.75°A
is -77.75°A
UFP of
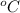 is -33.35°A
is -33.35°A
Normal boiling point of water =
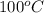
Putting values in above equation, we get:
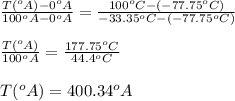
Hence, the boiling point of water is