Answer
The percent yield for the reaction = 81.88%
Step-by-step explanation
Given:
Mass of CuCl2 = 15 grams
Mass of NaNO3 = 20 grams
Actual yield of NaCl = 11.3 grams
Equation: CuCl₂ + 2NaNO₃ ----> Cu(NO₃)₂ + 2NaCl
What to find:
The percent yield for the reaction.
Step-by-step solution:
Step 1: Covert the given grams to moles.
Molar mass of CaCl₂ = 110.98 g/mol
Molar mass of NaNO₃ = 84.995 g/mol
The mass of the reactants can be converted to moles using the mole formula
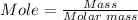
Moles of CaCl₂
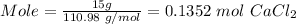
Moles of NaNO₃ is
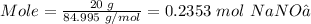
Step 2: Determine the limiting reactant.
From the equation, 1 mole CaCl₂ required 2 moles NaNO₃
So 0.1352 moles CaCl₂ will require (0.1352 x 2)/1 = 0.2704 moles NaNO₃
Note that the moles present in 20 grams NaNO₃ is 0.2353 mol, but 0.2704 mol NaNO₃ is needed to consume all the 0.1352 mol CaCl₂. This implies NaNO₃ is the limiting reactant and CaCl₂ is the reactant in excess.
Step 3: Calculate the theoretical yield for the reaction.
From the equation, 2 moles NaNO₃ produce 2 moles NaCl
Therefore 0.2353 moles NaNO₃ will produce
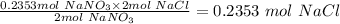
Step 4: Convert 0.2353 mol NaCl to mass.
Molar mass of NaCl = 58.44 g/mol
So 0.2353 mol = Mass/58.44 g/mol
Mass = 0.2353 mol x 58.44 g/mol
Mass of NaCl = 13.8 grams
Step 3: Determine the percent yield of the reaction.
The percent yield of the reaction can be calculated using the formula
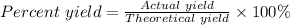
Actual yield of NaCl = 11.3 grams
Theoretical yield of NaCl = 13.8 grams
Thus,
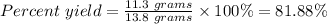
The percent yield for the reaction = 81.88%