Answer : The correct options are,
The reaction is endothermic.
The change in enthalpy is positive.
Explanation :
Endothermic reaction : It is defined as the chemical reaction in which the energy is absorbed from the surrounding.
In the endothermic reaction, the energy of reactant are less than the energy of product.
Exothermic reaction : It is defined as the chemical reaction in which the energy is released into the surrounding.
In the exothermic reaction, the energy of reactant are more than the energy of product.
Enthalpy change : It is the difference between the energy of product and the reactant. It is represented as
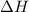 .
.
In an endothermic reaction, the energy of products are higher than the reactants energy. Thus, the change in enthalpy is positive.
In an exothermic reaction, the energy of products are lesser than the reactants energy. Thus, the change in enthalpy is negative.
Hence, the correct options are,
The reaction is endothermic.
The change in enthalpy is positive.