ANSWER
The percentage error is 13.04%
Step-by-step explanation
Given that;
Exact value = 1.15 g/ml
Approximate value = 1.00 g/ml
Follow the steps below to find the percentage error of water
Step 1; Apply the percentage error formula
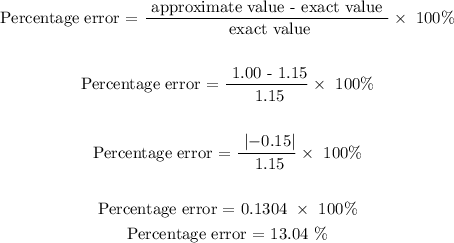
Therefore, the percentage error is 13.04%