Answer:
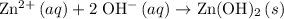 .
.
Step-by-step explanation:
Ionic Equation for this reaction
Rewrite only the species that exist as ions. Those species typically include:
- soluble salts,
- strong acids, and
- soluble bases.
In this reaction, both
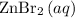 and
and
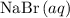 are salts. The state symbol "
are salts. The state symbol "
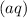 " suggests that both of these salts are soluble. Hence, both of these salts exist as ions and should be rewritten:
" suggests that both of these salts are soluble. Hence, both of these salts exist as ions and should be rewritten:
- Each
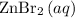 formula unit would exist as one
formula unit would exist as one
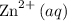 and
and
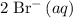 . Notice how there are twice as many
. Notice how there are twice as many
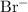 ions as
ions as
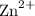 ions.
ions. - Each
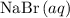 formula unit would exist as one
formula unit would exist as one
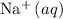 and one
and one
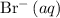 .
.
Similarly, the state symbol "
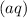 " suggests that the base
" suggests that the base
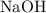 is also soluble:
is also soluble:
- Each
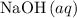 formula unit would exist as one
formula unit would exist as one
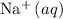 and one
and one
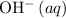 .
.
On the other hand, the state symbol "
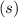 " suggests that the base
" suggests that the base
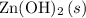 is a precipitate and is not soluble. Rather, the bonds within
is a precipitate and is not soluble. Rather, the bonds within
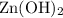 stay mostly intact, and this species would not exist as ions. Hence, do not rewrite
stay mostly intact, and this species would not exist as ions. Hence, do not rewrite
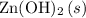 when deriving the ionic equation for this reaction.
when deriving the ionic equation for this reaction.
Hence, the ionic equation for this reaction would be:
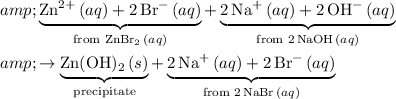 .
.
Net Ionic Equation for this reaction
Eliminate species that are present on both sides of the ionic equation to obtain the net ionic equation. A species should be eliminated if only if an equal number of this species are found on both sides of the ionic equation. Otherwise, subtract from the side with a larger number of that species.
For this reaction, the net ionic equation would be:
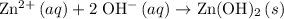 .
.