INFORMATION:
We have the next graph
And we must add the label for the activation energy
STEP BY STEP EXPLANATION:
To know where is the activation energy in the graph, we need to know that:
- Activation energy: It is defined as the minimum amount of energy absorbed by the reactant molecules so that their energy becomes equal to the threshold energy.
The formula for activation energy is
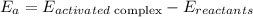
Where,
- An activated complex is an intermediate state that is formed during the conversion of reactants into products. An activated complex is the structure that results in the maximum energy point along the reaction path.
- The energy of reactants is the initial part of the graph.
Finally, since the activation energy is the difference between activated complex (maximum energy point) and the energy of reactants, we can state that the activation energy label would be located in letter B.
ANSWER:
D. B