Assuming the "compound" is the KCl, we can use the mass given to calculate the number of moles of KCl and, using it we can calcualte the volume that contains that number of moles of KCl.
The molar concentration is given by:
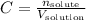
Since we want to find the volume of the solution, we can solve by it:
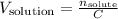
The number of moles of solute is the number of moles of KCl in the solution, so:
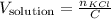
We know that concentration, so we only need the number of moles. For this, we will need the molar mass of KCl.
Since we have 1 K and ' Cl on KCl, its molar mass is the sum of the atomic masses of K and Cl:
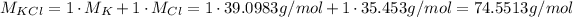
Usin it and the mass that we have, we can calculate the number of moles contained in the given mass:
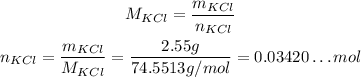
Now that e know the number of moles of KCl, we can input into the other equation to calculate the volume of solution:
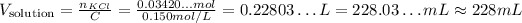
So, the volume is approxiamtely 228 mL