Answer
The empirical formula for the compound is C₂H₄O₁
Step-by-step explanation
Given:
Mass of sample = 6.50 g
Mass of C = 3.54 g
Mass of H = 0.59 g
Mass of O = Mass of sample - (Mass of C + Mass of H) = 6.50 g - (3.54 g + 0.59 g) = 2.37 g
What to find:
The empirical formula for the compound.
Step-by-step solution:
Step 1: Determine the mole of each element present.
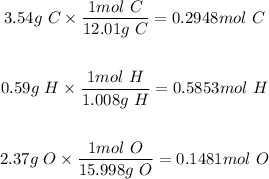
Step 2: Divide each mole by the smallest number of moles (0.1481 mol)
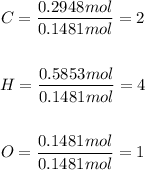
Step 3: Determine the empirical formula for the compound by using the mole ratio as the subscript.
Therefore, the empirical formula for the compound is:
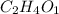
The empirical formula for the compound is C₂H₄O₁