Answer:
D. spectator ions.
Step-by-step explanation:
Hello!
In this case, when going over net ionic equations by which precipitation reactions are analyzed, we can consider the example of lead (II) nitrate with potassium iodide to yield insoluble lead (II) iodide and soluble potassium nitrate according to:
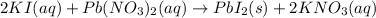
Whereas aqueous species remain in solution:
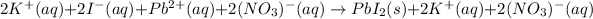
It means that potassium and nitrate ions are spectator ions because they are not involved in the precipitation reaction, which is represented by the net ionic one:
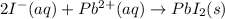
Thus, the answer to this question is D. spectator ions.
Best regards!