Given:
The mole fraction of Na2S = 0.125
Molar mass of Na2S = 78.04 g/mol
Molar mass of water = 18.016 g/mol
We want to know the weight/weight % of Na2S
Answer:
To answer this question, we will use the mole fraction of Na2S to find the total mass of the sample and the mass of the solute.
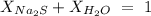
We will use the above equation, assuming that the solution containes Na2S as the solute and H2O as the solvent.
Since we have 0.125 mole fraction, this means we have 0.125 moles of Na2S per mole of solution. Therefore the mass of Na2S and H2O will be:
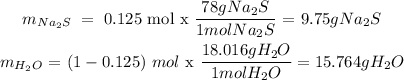
After knowing the masses of both the solute and the solvent, we can the calculate the weight/weight% like this:
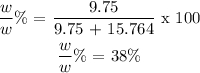
Therefore the answer is 38%.