we need to cancel the units
b/c would be equal to 1 to retain the same amount, remember, 1a=a
so
b/c=1mol/44g or 44g/1mol
alrighty
we waant to convert 27.8mols
so therefor a=27.8mol
27.8mol times b/c=D
we want c to be mols and b to be grams so the mols cancel
so thefor c=1mol
it would look like this:
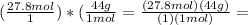
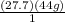
I would write 1 mol of carbon dioxide for C (choice A)