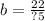Answer:
Solution:
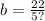
Explanation:
Rearrange terms:

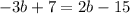
subtract 7 from both sides of the equation:
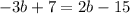
simplify:
subtract the numbers
⬇️
⬇️
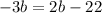
subtract ⬇️ from both sides of the equation
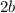
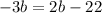
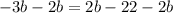
simplify:
⬇️combine like terms
⬇️combine like terms
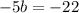
divide both sides of the equation by the same term:
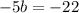
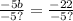
simplify:
⬇️cancel terms that are in both the numerator and the denominator
⬇️divide the numbers