Answer:
The molar mass of the gas is 43.5g/mol.
Step-by-step explanation:
The given information of the gas from the exercise is:
- Volume (V): 250mL (0.25L)
- Mass: 0.435g
- Pressure (P): 743mmHg (1atm)
- Temperature (T): 29°C (302K)
1st) We can use the Ideal Gases formula to calculate the number of moles of the gas, remember to convert the units to atm, liters and Kelvin:
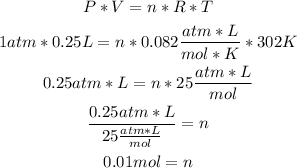
2nd) Now we can calculate the molar mass of the gas using the mass and the 0.01 moles, with a mathematical rule of three:
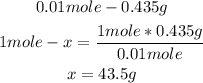
Finally, the molar mass of the gas is 43.5g/mol.