Answer: 4 moles of water are produced.
Number 2 is used to perform this calculation
Step-by-step explanation:
The balanced chemical equation is:
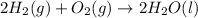
As it is given, excess oxygen is present, oxygen acts as excess reagent and hydrogen acts as limiting reagent as it limits the formation of products
According to stoichiometry:
2 moles of hydrogen give = 2 moles of water
Thus 4 moles of hydrogen give =
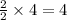 moles of water
moles of water
4 moles of water are produced . Thus the coefficient 2 from hydrogen and coefficient 2 from water that appear in the balanced chemical equation is used.