Answer:
It will read the same at F = -40.
Explanation:
The relation between fahrenheit and celsius is given by the following equation:
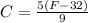
At what temperature will a Fahrenheit thermometer read the same as a Celsius thermometer?
This is F for C = F. So
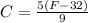
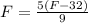
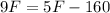
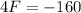
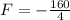
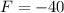
It will read the same at F = -40.