Answer:
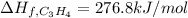
Step-by-step explanation:
Hello!
In this case, since the equation we use to model the heat exchange into the calorimeter and compute the heat of reaction is:

We plug in the mass of water, temperature change and specific heat to obtain:
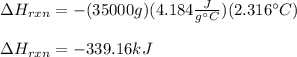
Now, this enthalpy of reaction corresponds to the combustion of propyne:
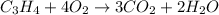
Whose enthalpy change involves the enthalpies of formation of propyne, carbon dioxide and water, considering that of propyne is the target:
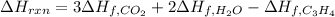
However, the enthalpy of reaction should be expressed in kJ per moles of C3H4, so we divide by the appropriate moles in 7.00 g of this compound:
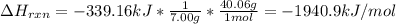
Now, we solve for the enthalpy of formation of C3H4 as shown below:
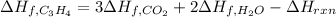
So we plug in to obtain (enthalpies of formation of CO2 and H2O are found on NIST data base):
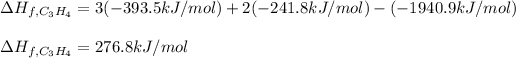
Best regards!