The question requires us to calculate the mass of sulfur (S8) necessary to obtain 23.2 L of sulfur dioxide (SO2), considering standard temperature and pressure.
To answer this question, we'll go through the following steps:
1) Calculate the amount of moles of gas that corresponds to 23.2 L of gas, considering STP;
2) Using stoichiometry, calculate how many moles of S8 would be necessary to obtain the amount calculated in step 1;
3) Using the molar mass of S8, convert the amount of moles calculated in step 2 to mass of S8.
Next, we'll go through each one of these steps:
1) At standard temperature and pressure conditions (STP), 1 mol of any gas corresponds to 22.4 L of this gas. Thus we can write:
22.4 L SO2 ------------------- 1 mol SO2
23.2 L SO2 ------------------ x
Solving for x, we'll have:
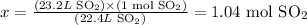
2) Now, we must use the stoichiometry of the balanced chemical equation given to calculate how many moles of S8 would be necessary to produce 1.04 moles of SO2:
8 mol SO2 ----------------------- 1 mol S8
1.04 mol SO2 ------------------ y
Solving for y, we'll have:
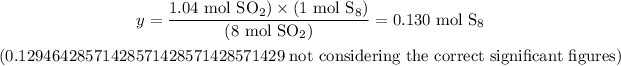
3) At last, we must use the molar mass of S8 to calculate how many grams of this compound are there in 0.129 moles of it. Since the atomic mass of sulfur (S) is 32.06 u, the molar mass of S8 is:
molar mass (S8) = 8 x 32.06 = 256.5 g/mol (or 256.48 g/mol, not considering the correct amoung of significant figures)
Knowing the molar mass of S8, we can write:
1 mol S8 ------------------------- 256.5 g S8
0.130 mol S8 ------------------ z
Solving for z, we'll have:
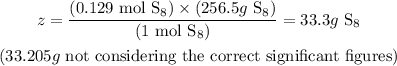
Therefore, the amount of S8 necessary to obtain 23.2 L of SO2 at STP is 33.3g (33.205g not considering the correct significant figures throughout the entire calculation).