Answer:
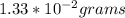
Explanations
The complete balanced equation for the given reaction is expressed as;
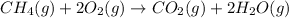
Given the following parameters
Mass of CH4 = 5.90×10^−3 g = 0.0059grams
Determine the moles of methane
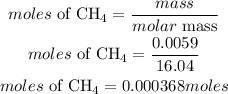
According to stoichimetry, 1 mole of methane produces 2 moles of water, hence the moles of water required will be:
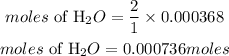
Determine the mass of water produced
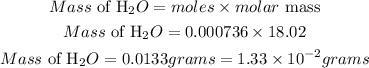
Therefore the mass of water produced from the complete combustion of 5.90×10−3 g of methane is 1.33 * 10^-2grams