Step-by-step explanation:
We are given: mass of PbS = 393g
: mass of PbO = 593g
We know: molar of PbS = 239.3g/mol
: molar mass of PbO = 223.2g/mol
: molar mass of Pb = 207.2g/mol
m is mass and M is molar mass.
We first determine the mass of Pb from the mass of PbS:
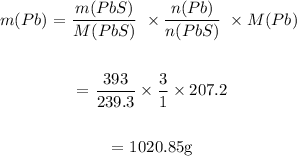
We then determine the mass of Pb from the mass of PbO:
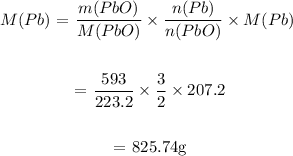
From the masses obtained above, it is clear to observe that PbO is the limiting reactant.
Answer:
mass of Pb = 826g