Answer:
The percent of oxalic acid in a solid is 48.87%.
Step-by-step explanation:
Mass of the solid sample = 0.7984 g
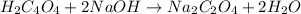
Volume of NaOH solution = 37.98 mL = 0.03798 L
Concentration or molarity of the NaOH solution = 0.2283 M
Moles of NaOH :
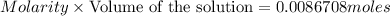
According to reaction, 2 moles of sodium hydroxide reacts with 1 mole of oxalic acid .
Then 0.0086708 moles of sodium hydroxide will react with:
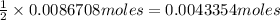 of oxalic acid.
of oxalic acid.
Mass of oxalic acid neutralized = 0.0043354 moles × 90 g/mol =0.390186 g
Percentage of oxalic acid in solid sample :
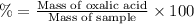
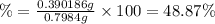
The percent of oxalic acid in a solid is 48.87%.