Step 1
Oxygen gas is considered to be ideal.
Therefore, it is applied:
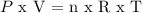
--------------------
Step 2
Information provided:
R = 8.314 m3
P = 36.0 kPa = 36.0 x (1000 Pa/1 kPa) = 36000 Pa
V = 38 L x (1 m3/1000 L) = 0.038 m3
T = 82 °C + 273 = 355 K
Procedure:
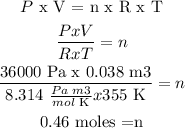
Answer: n = 0.46 moles