Molarity has the following equation:
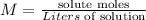
In this case, we need to find the solute mass given that the liters of solution are 2.1L and the molarity of the solution is 0.23 molar. Replacing:
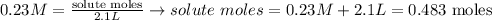
We have 0.483 moles of solute. To find the mass, we multiply by the molar mass:
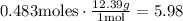
Therefore, there are 5.98g of solute.