The first step to solve this question is to write and balance the chemical equation that describes the reaction:
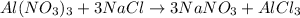
According to the given information the limiting reactant is NaCl, this is because 9 moles of this reactant react with 3 moles of Al(NO₃)₃.
Use this information to find the maximum amount of NaNO₃ that was produced:
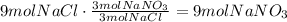
It means that 9 moles of NaNO₃ are produced.