The question requires us to calculate the mass, in grams, contained in 5.26 x 10^-4 mol of HC2H3O2.
To solve this question, first we need to calculate the molar mass of the compound, considering the number of atoms of each element, and then relate the value obtained with the number of moles given (5.26 x 10^-4 mol).
First, to calculate the molar mass of the compound, let's consider the following atomic masses:
atomic mass of C = 12.01 u
atomic mass of H = 1.007 u
atomic mass of O = 15.99 u
Next, we calculate the molar mass. To do that, we need to consider the number of atoms of each element: according to the chemical formula, there are 2 atoms of C, 4 atoms of H and 2 atoms of O:
molar mass (C2H4O2) = (2 * 12.01) + (4 * 1.007) + (2 * 15.99) = 60.03 g/mol
Now, we know that there are 60.03 g for each mol of the compound. With that information, we can estabilish the following relation to calculate the mass contained in 5.26 x 10^-4 mol of the compound:
1 mol --------------------- 60.03 g
5.26 x 10^-4 mol ----- x
Solving for x, we have:
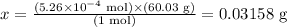
Therefore, there are 0.03158 g of HC2H3O2 in 5.26 x 10^-4 mol of this compound.