The question provides the chemical reaction between NaCl and H2SO4 and requires us to balance the reaction and identify the type of reaction the equation represents.
The chemical reaction as provided by the question is as it follows:
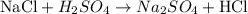
where a salt (NaCl) reacts with a strong acid (H2SO4) to produce another salt (Na2SO4) and acid (HCl).
Note that, we can rewrite the reaction as AB + CD -> AD + CB, where A corresponds to Na+, B corresponds to Cl-, C corresponds to H+ and D corresponds to (SO4)2-.
This type of representation of a chemical reaction (AB + CD -> AD + CB) indicates a double displacement reaction, in which the positive and negative ions of two ionic compounds exchange places to form two new compounds. Thus, as we can identify all elements of the reaction in this representation, we can say that it is a double displacement reaction.
Next, we need to balance the reaction given. A simple method to balance a chemical reaction is follow the order: metals > non-metals > carbon > hydrogen > oxygen
- First, we adjust the amount of atoms of Na, a metal. We can see that there is only 1 Na atom on the left side and there are 2 atoms on the right side; thus, we can adjust the coefficient of NaCl from 1 to 2 to achieve the same number of Na atoms on both sides:
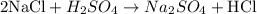
- Next, we need to consider Cl. We can see that, at this point, there are 2 Cl atoms on the left side and only 1 Cl atom on the right side; thus, we can adjust the coefficient of HCl from 1 to 2 to adjust the amount of Cl atoms:
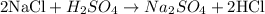
- Now, moving on to S, we can see that there is the same amount of S atoms on both sides, thus we don't need to change the coefficients to adjust it.
- The next atom we need to consider is H. We can see 2 atoms on the left side (in the H2SO4 molecule) and 2 H atoms on the right side (2 * HCl molecules), thus we don't need to change the coefficients to adjust the amount of H atoms.
- At last, we must check the O atoms. We can see 4 atoms on the left side (in H2SO4) and 4 O atoms on the right side (in Na2SO4), thus we don't need to adjust the amount of O atoms.
Therefore, the balanced reaction is:
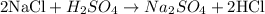
To summarize the answer, we can say that the coefficients of the balanced chemical reaction are 2, 1, 1 and 2 for NaCl, H2SO4, Na2SO4 and HCl, respectively, and that the reaction given is a double displacement reaction.