Answer:
Polar covalent.
Step-by-step explanation:
Hello,
In this case, ionic and covalent bonds could be differentiated by subtracting the constituents' electronegativities. Thus, for iron and chlorine, we have:
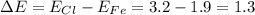
Hence, when such difference is less than 1.7, the bond is polar covalent as it is also greater than 0.7.
Best regards.