Answer
The percent mass per volume of the solution = 2.4%
Step-by-step explanation
Given that:
Mass of dextrose in the solution = 6.00 g
Volume of solution = 2.50 x 10² mL
What to find:
To calculate the percent mass per volume of the solution.
Step-by-step solution:
To calculate the percent mass per volume of the solution, we use:
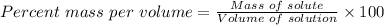
Substitute the parameters given into the formula, the % (m/v) is
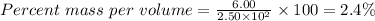
The percent mass per volume, % (m/v) of the solution is 2.4%.