Answer : The number of atoms in a 4.0 mole sample of argon has greater number of atoms or four times as compared to the number of atoms in a 1.0 mole sample of neon.
Explanation :
As we know that the 1 mole of substance contains
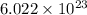 number of atoms.
number of atoms.
As per question,
The 1 mole sample of neon contains
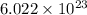 number of atoms.
number of atoms.
And,
As, 1 mole sample of argon contains
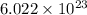 number of atoms
number of atoms
So, 4.0 mole sample of argon contains
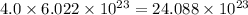 number of atoms
number of atoms
From this we conclude that, the number of atoms in a 4.0 mole sample of argon has greater number of atoms or four times as compared to the number of atoms in a 1.0 mole sample of neon.