Answer: The correct answer is atomic number, mass number.
Step-by-step explanation:
Isotopes are defined as the chemical species which have same number of protons but differ in the number of neutrons.
It can also be said that they are the chemical species of the same element which have same atomic number but have different mass number.
For Example:
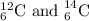 are the isotopes of carbon element, which have same atomic number but different mass number.
are the isotopes of carbon element, which have same atomic number but different mass number.
Hence, the correct answer is atomic number, mass number.