Answer : The correct option is, 4.81 g
Explanation : Given,
Mass of aluminium = 2.70 g
Molar mass of aluminium = 26.98 g/mole
Molar mass of sulfur = 32.07 g/mole
First we have to calculate the moles of aluminium.
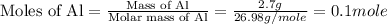
Now we have to calculate the moles of sulfur.
According to the question,
As, 2 moles of aluminium react with 3 moles of sulfur
So, 0.1 mole of aluminium react with
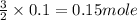 of sulfur
of sulfur
Now we have to calculate the mass of sulfur.
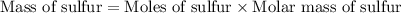
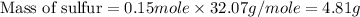
Therefore, the mass of sulfur he take must be, 4.81 g