Step-by-step explanation:
According to the the ideal gas law, PV= nRT.
This means that pressure is directly proportional to temperature.
So, when HCl and zinc are combined in a flask then it will lead to the formation of zinc chloride and hydrogen gas.
The reaction equation will be as follows.
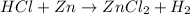
Since, the flask is sealed hence, hydrogen gas will not be able to move out of the flask.
So, when it will behave ideally then due to directly proportional relation between pressure and temperature there will occur a decrease in temperature with decrease in pressure.