Answer: The element having given valence electronic configuration is beryllium.
Step-by-step explanation:
Electronic configuration is defined as the representation of electrons around the nucleus of an atom. Number of electrons in an atom are determined by the atomic number of that atom.
Valence electrons are defined as the electrons present in the outermost shell of an atom.
We are given:
Valence electronic configuration of an atom =
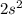
So, the actual electronic configuration of atom will be
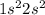
Total number of electrons = 2 + 2 = 4
So, the atomic number of given element is 4 and the element is beryllium
Hence, the element having given valence electronic configuration is beryllium.