Answer:
density=1.2g/cm^3
Step-by-step explanation:
Hello,
Density is defined as the degree of compactness of a substance and is mathematically expressed via the division between the substance's mass and the occupied volume:
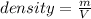
Thus, the occupied volume is:

Finallt, the computed density:
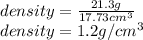
Best regards.