Step-by-step explanation:
Scientists most especially chemists use the mole concepts through balanced chemical equations and molar masses to represent chemical reactions and account for the amount of substances.
The mole concept entails finding molar relationships between reactants and products in a chemical reaction. Mole connects difference chemical parameters and it is used in quantitative analysis of chemical reaction.
- First, we work from the known to the unknown.
- The known is the specie whose mass or number of moles is given.
Number of moles =
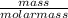
- After obtaining the number of moles, then go ahead to compared using the balanced reaction with that of the unknown.
- Then from them find the mass of the unknown.