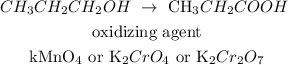Answer:
Step-by-step explanation:
Here, we want to write an equation of reaction that produces propanoic acid from 1-propanol
1-propanol can be converted to propanoic acid with the use of strong oxidizing agents. These strong oxidizing agents help in the conversion of propanol to propanoic acid.
Examples of these strong oxidizing agents include potassium permanganate (KMnO4), Potassium chromate (K2Cr2O4) or potassium Dichromate (K2Cr2O7).
We have the equation of reaction as follows: