Answer:
The value of y is 18 at x = 70
Explanation:
The equation of the directly proportional is y = k x, where k is the constant of proportionality.
∵ The value of y is directly proportional to the value of x
→ By using the equation above
∴ y = k x
∵ x = 140 and y = 36
→ Substitute them in the equation above
∴ 36 = k(140)
∴ 36 = 140k
→ Divide both sides by 140
∵
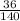 = k
= k
∴
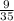 = k
= k
→ Substitute the value ok in the equation
∴ y =
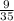 x ⇒ equation of proportionality
x ⇒ equation of proportionality
∵ x = 70
→ Substitute in the equation to find y
∴ y =
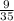 × 70
× 70
∴ y = 18
∴ The value of y is 18 at x = 70